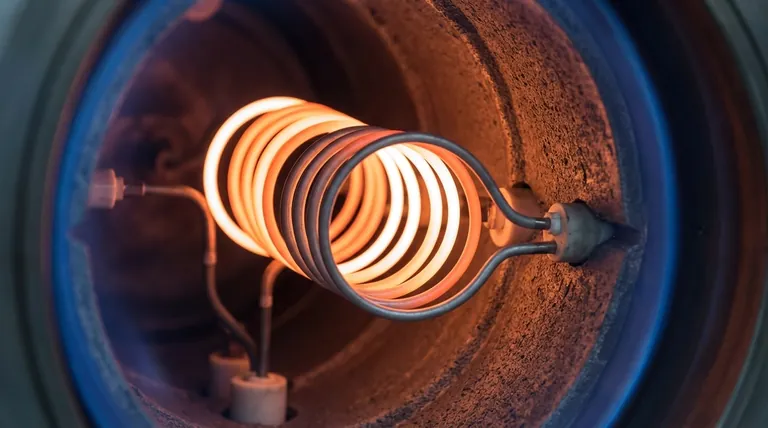
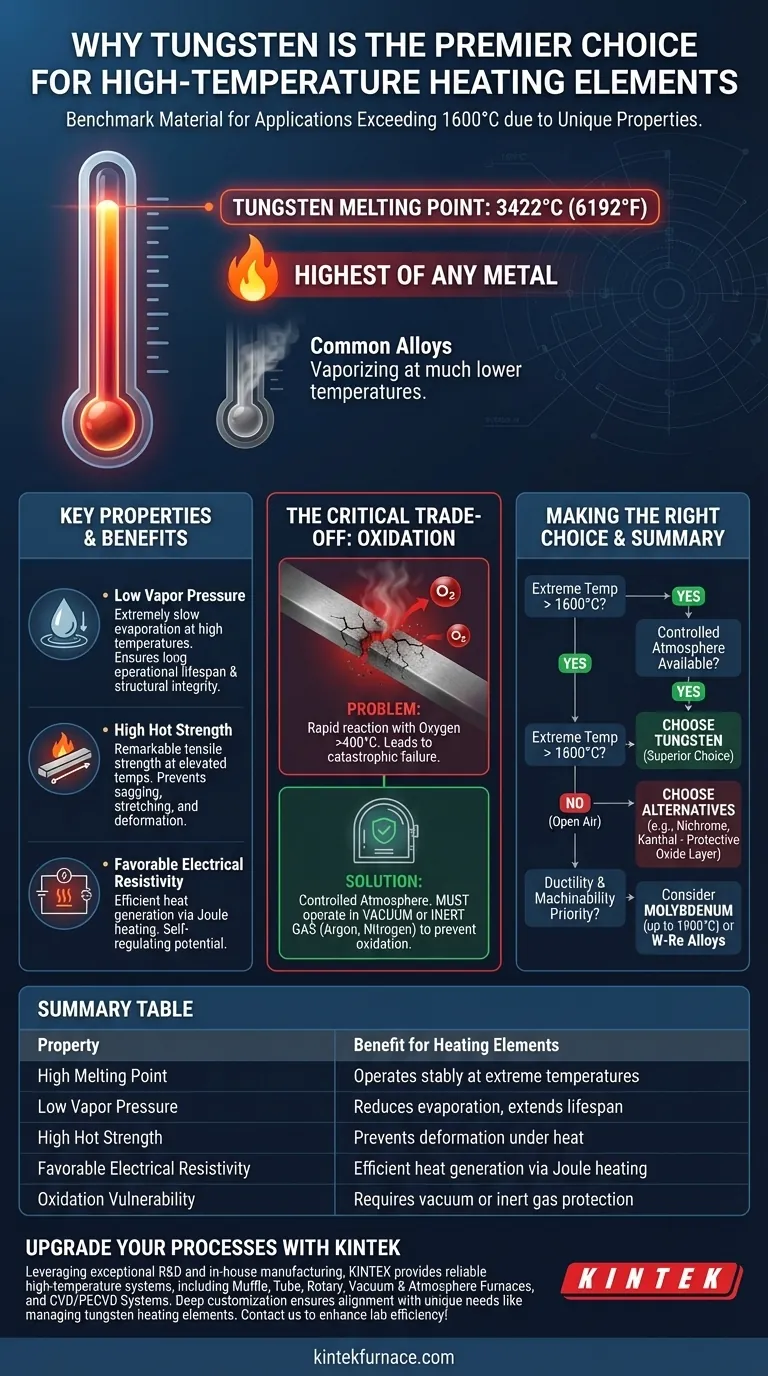
At its core, tungsten is suitable for high-temperature heating elements because it has the highest melting point of any metal, allowing it to remain solid and stable at temperatures where most other materials would have already vaporized. This fundamental property makes it the benchmark material for applications exceeding 1600°C.
While its high melting point is the headline feature, tungsten's true value comes from a combination of properties: high-temperature strength, low vapor pressure, and suitable electrical resistance. However, these benefits can only be realized when its critical weakness—oxidation—is managed through a controlled atmosphere.
Why Tungsten Excels Under Extreme Heat
To understand why tungsten is the preferred choice, we must first define the ideal characteristics of a high-temperature heating element. The material must not only survive the heat but also perform reliably over a long service life.
The Highest Melting Point
Tungsten’s melting point of 3422°C (6192°F) is its most critical advantage. This incredibly high threshold allows it to operate effectively in vacuum furnaces and other applications that require sustained, extreme heat far beyond the capacity of common alloys.
Low Vapor Pressure
At high temperatures, atoms on a material's surface can sublimate, or turn directly into a gas. Tungsten has a very low vapor pressure, meaning it evaporates extremely slowly even when white-hot. This ensures the heating element maintains its mass and structural integrity for a longer operational lifespan.
High Hot Strength
Many metals become soft and weak long before they melt. Tungsten, by contrast, possesses remarkable tensile strength at elevated temperatures. This "hot strength" prevents the element from sagging, stretching, or deforming under its own weight, which is crucial for maintaining a consistent shape and function.
Favorable Electrical Resistivity
A heating element works by converting electrical energy into heat through resistance (Joule heating). Tungsten’s electrical resistivity is high enough to generate significant heat efficiently but not so high that it becomes difficult to pass current through it. Its resistivity also increases with temperature, which can help in self-regulating designs.
Understanding the Critical Trade-off: Oxidation
Tungsten's remarkable properties come with one major vulnerability that dictates how and where it can be used. Ignoring this limitation leads to rapid and catastrophic failure.
The Problem with Oxygen
Despite its heat resistance, tungsten reacts readily with oxygen at high temperatures. This process, oxidation, begins around 400°C and accelerates rapidly, forming a volatile tungsten oxide that causes the element to quickly disintegrate.
The Solution: A Controlled Atmosphere
Because of its high reactivity with oxygen, a tungsten heating element cannot be operated in open air. To function correctly, it must be protected inside a vacuum or surrounded by a non-reactive, inert gas such as argon or nitrogen. This design constraint is fundamental to any system using tungsten elements.
Making the Right Choice for Your Application
Selecting the right material requires balancing performance goals with environmental constraints. Tungsten's properties make it a specialist material, not a universal solution.
- If your primary focus is reaching extreme temperatures (above 1600°C): Tungsten is the superior choice, provided your design can incorporate a vacuum or an inert gas atmosphere to prevent oxidation.
- If your application must operate in open air at high temperatures: You must use an alternative like a nickel-chromium alloy (Nichrome) or Kanthal (iron-chromium-aluminum), which form a protective oxide layer.
- If ductility and machinability are your top priorities: Consider molybdenum for temperatures up to around 1900°C, or specialized tungsten-rhenium alloys that improve ductility at a higher cost.
Ultimately, successful high-temperature design depends on selecting a material whose strengths align with your operational environment and whose weaknesses can be effectively managed.
Summary Table:
| Property | Benefit for Heating Elements |
|---|---|
| High Melting Point (3422°C) | Operates stably at extreme temperatures |
| Low Vapor Pressure | Reduces evaporation, extends lifespan |
| High Hot Strength | Prevents deformation under heat |
| Favorable Electrical Resistivity | Efficient heat generation via Joule heating |
| Oxidation Vulnerability | Requires vacuum or inert gas protection |
Upgrade your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable high-temperature systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, such as managing tungsten heating elements in controlled environments. Contact us today to discuss how our expertise can enhance your lab's efficiency and performance!
Visual Guide
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What are the primary applications of MoSi2 heating elements in research? Achieve Reliable High-Temp Control for Material Synthesis
- What are the advantages of using molybdenum-disilicide heating elements for aluminum alloy processing? (Rapid Heating Guide)
- What is the temperature range for MoSi2 heating elements? Maximize Lifespan in High-Temp Applications
- What ceramic materials are commonly used for heating elements? Discover the Best for Your High-Temp Needs
- What is the temperature range where MoSi2 heating elements should not be used for long periods? Avoid 400-700°C to Prevent Failure
















