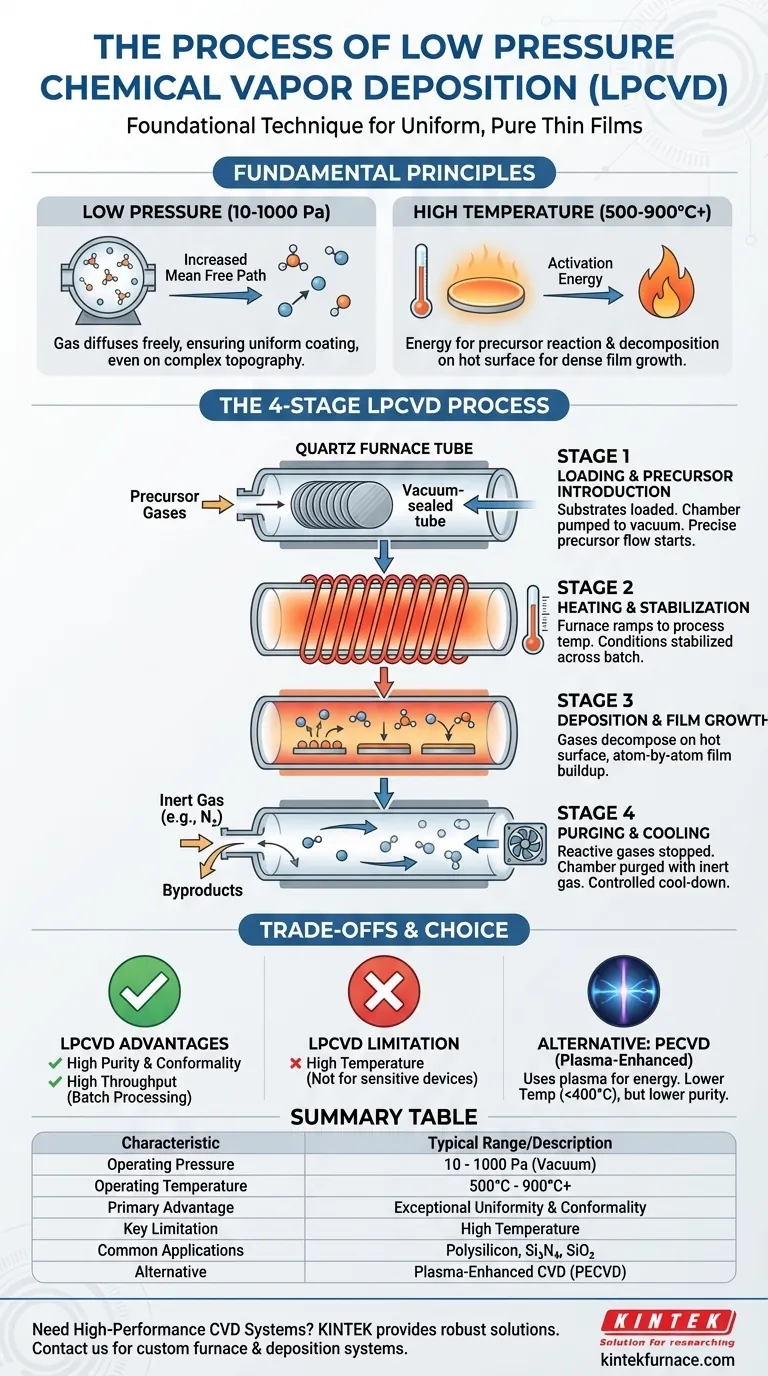
In essence, the process of Low Pressure Chemical Vapor Deposition (LPCVD) involves four key stages. First, precursor gases are introduced into a vacuum chamber containing the material to be coated (the substrate). Second, the chamber is heated to a high temperature, providing the energy needed for a chemical reaction. Third, these gases react and decompose on the hot substrate surface, forming a solid, highly pure thin film. Finally, the chamber is purged of unreacted gases and cooled down.
Low Pressure Chemical Vapor Deposition is a foundational technique in semiconductor manufacturing prized for one primary reason: control. By combining high heat with very low pressure, LPCVD enables the growth of exceptionally uniform and pure thin films, even over large batches of substrates with complex surface topographies.

The Fundamental Principles of LPCVD
To truly understand the process, we must look at the two core environmental conditions that define it: low pressure and high temperature. These are not arbitrary settings; they are precisely controlled to achieve specific material properties.
Why Low Pressure is Critical
The "low pressure" aspect of LPCVD is key to its most significant advantage: uniformity. Operating in a vacuum (typically 10-1000 Pa) dramatically increases the mean free path of gas molecules.
This means gas particles travel much farther before colliding with each other. As a result, they can diffuse more freely and evenly throughout the chamber, coating all surfaces of the substrate—and even multiple vertically stacked substrates—with exceptional consistency.
The Role of High Temperature
LPCVD is a thermally-driven process. The high temperatures, often ranging from 500°C to over 900°C, provide the activation energy required for the precursor gases to chemically react and break down.
This reaction happens primarily on the hot substrate surface, not in the gas phase. This surface-controlled reaction is what allows for the slow, orderly, atom-by-atom growth of a dense and high-quality film.
What are Precursor Gases?
Precursors are the building blocks of the film. They are volatile chemical compounds that contain the elements you wish to deposit.
For example, to deposit a film of silicon nitride (Si₃N₄), one might use dichlorosilane (SiH₂Cl₂) and ammonia (NH₃) as precursor gases. At high temperatures, these gases react to form solid silicon nitride on the substrate, with the gaseous byproducts being pumped away.
The Four Stages of the LPCVD Process
The LPCVD process is executed in a highly controlled, automated sequence inside a furnace, typically a long quartz tube.
Stage 1: Loading and Precursor Introduction
Wafers or other substrates are loaded into the furnace. The chamber is then sealed and pumped down to its target low pressure. Once the vacuum is stable, a precise flow of precursor gases is introduced into the chamber.
Stage 2: Heating and Stabilization
The furnace ramps up to the exact process temperature. This temperature must be held incredibly steady—often within a fraction of a degree—across the entire length of the furnace to ensure every substrate experiences identical conditions for uniform film growth.
Stage 3: Deposition and Film Growth
With the temperature and gas flow stable, the deposition begins. Precursor gases decompose on the hot substrate surfaces, gradually building the desired thin film. This stage can last from minutes to hours, depending on the material and desired thickness, which can range from a few nanometers to several micrometers.
Stage 4: Purging and Cooling
Once the target thickness is reached, the flow of reactive precursor gases is stopped. An inert gas, such as nitrogen, is used to purge the chamber, removing any unreacted gases and reaction byproducts. The furnace then begins a controlled cool-down sequence before the finished substrates can be safely removed.
Understanding the Trade-offs
LPCVD is a powerful and widely used technique, but it is not the solution for every application. Understanding its advantages and limitations is key to using it effectively.
Advantage: Film Purity and Conformality
Because LPCVD is a purely thermal process, it produces films with very high purity and low internal stress. Its ability to uniformly coat complex, high-aspect-ratio trenches and structures—a property known as conformality—is outstanding and a primary reason for its use.
Advantage: High Throughput
LPCVD furnaces are batch systems, capable of processing 100 to 200 wafers simultaneously. This makes the process extremely cost-effective for high-volume manufacturing of foundational layers like polysilicon, silicon nitride, and silicon dioxide.
Limitation: High Temperature
The primary drawback of LPCVD is its high operating temperature. These temperatures can damage or alter previously fabricated structures on a device, such as aluminum interconnects. This makes LPCVD unsuitable for deposition steps that occur late in the manufacturing process.
Alternative: Plasma-Enhanced CVD (PECVD)
For temperature-sensitive applications, Plasma-Enhanced CVD (PECVD) is often used. PECVD uses an electric field to create plasma, which provides the energy for the reaction. This allows deposition to occur at much lower temperatures (typically < 400°C), but often at the cost of lower film purity and conformality compared to LPCVD.
Making the Right Choice for Your Goal
Selecting the correct deposition method requires matching the process capabilities to the material requirements and device constraints.
- If your primary focus is ultimate purity and uniform coverage on complex topographies: LPCVD is the superior choice for thermally stable substrates.
- If your primary focus is depositing a film on a temperature-sensitive device: A lower-temperature process like PECVD is the necessary alternative.
- If your primary focus is cost-effective, high-volume production of foundational films: The batch-processing capability of LPCVD makes it an economic powerhouse.
Understanding these core principles allows you to move beyond simply knowing the steps of a process to making informed engineering decisions.
Summary Table:
| Key LPCVD Process Characteristic | Typical Range / Description |
|---|---|
| Operating Pressure | 10 - 1000 Pa (Vacuum) |
| Operating Temperature | 500°C - 900°C+ |
| Primary Advantage | Exceptional Film Uniformity & Conformality |
| Key Limitation | High Temperature (Not suitable for temperature-sensitive substrates) |
| Common Applications | Polysilicon, Silicon Nitride, Silicon Dioxide deposition |
| Alternative for Low-Temp Needs | Plasma-Enhanced CVD (PECVD) |
Need a High-Performance LPCVD or PECVD System for Your Lab?
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides semiconductor and advanced materials laboratories with robust, high-temperature furnace solutions. Our product line, including Tube Furnaces, CVD/PECVD Systems, and Vacuum & Atmosphere Furnaces, is complemented by our strong deep customization capability to precisely meet your unique experimental requirements—whether you need ultimate film purity with LPCVD or lower-temperature deposition with PECVD.
Contact our experts today to discuss how we can tailor a solution for your thin film deposition challenges.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What is the significance of the cold wall CVD technique in graphene research? Unlock Precision Growth for High-Quality Graphene
- What advanced materials can be produced using CVD? Explore High-Performance Coatings and Nanostructures
- What is the primary function of a sputtering deposition system in graphene growth? Expert Catalyst Engineering
- How do CVD coatings compare to spray-on PTFE coatings? Discover Superior Performance and Safety
- What is an example of chemical vapor deposition? Building the Microchips in Your Electronics
- What is the function of a customized spray pyrolysis chamber? Optimize ZnSe and PbSe Thin Film Synthesis
- What are the key characteristics of CVD furnaces? Unlock Precision Thin Film Deposition
- What are the advantages of CVD vs. powder methods for catalysts? Unlock binder-free, high-performance electrode growth.



















