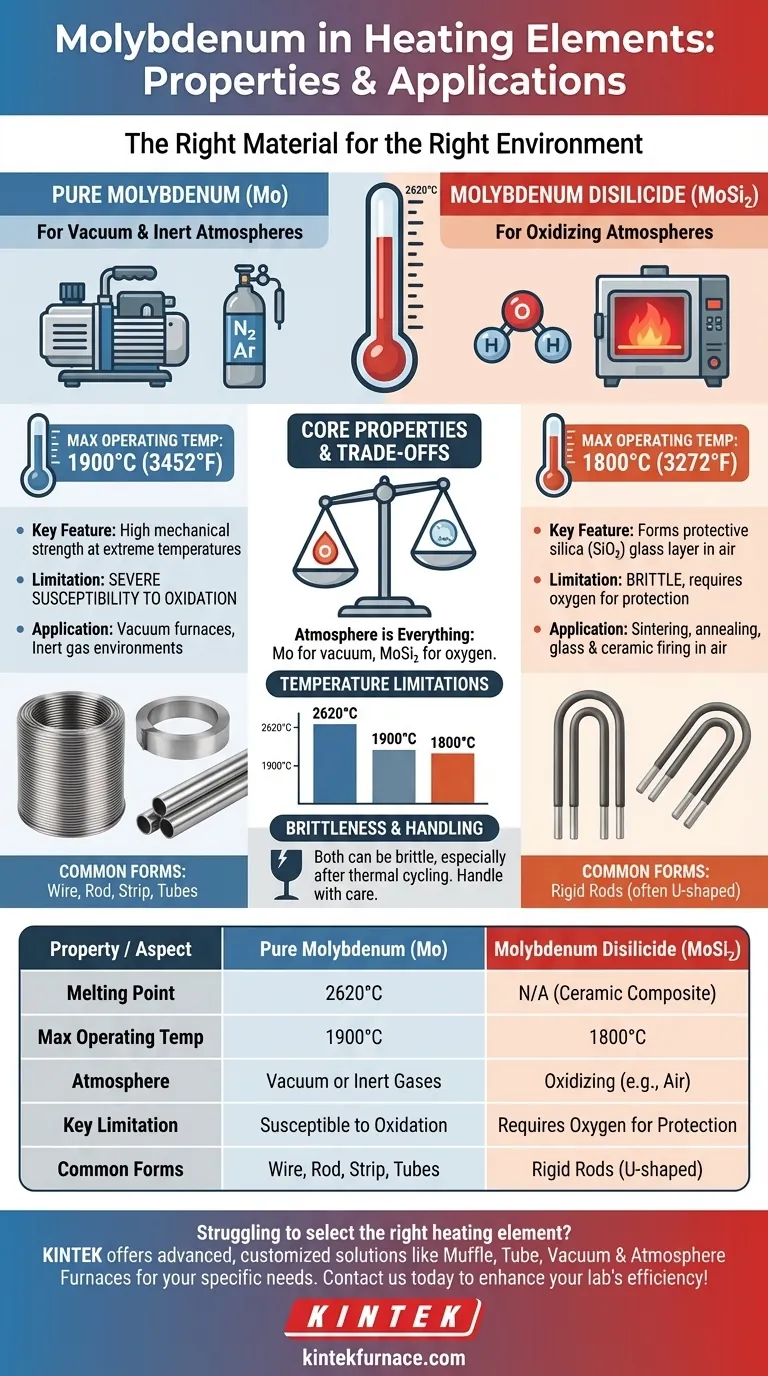
In high-temperature applications, Molybdenum is a critical material for heating elements due to its exceptional heat resistance and structural stability. Pure Molybdenum (Mo) and Molybdenum Disilicide (MoSi₂) serve distinct roles based on the furnace atmosphere, with pure Mo suited for vacuum environments and MoSi₂ designed for operation in oxidizing atmospheres like air.
The choice between Molybdenum-based heating elements is not about one being better, but about matching the material to its operating environment. Pure Molybdenum is for vacuum or inert gas furnaces, while Molybdenum Disilicide is for high-temperature applications in the presence of oxygen.

Understanding the Two Types of Molybdenum Elements
Molybdenum's utility in heating is not monolithic. It is crucial to distinguish between two primary forms: pure Molybdenum (Mo) and Molybdenum Disilicide (MoSi₂), as they have fundamentally different operating principles and applications.
Pure Molybdenum (Mo) Elements
Pure Molybdenum is a refractory metal with an extremely high melting point of approximately 2620°C (4748°F). This inherent thermal stability makes it an excellent choice for constructing heating elements.
Its key advantage is maintaining high mechanical strength at extreme temperatures, allowing it to function where other metals would fail.
However, its primary limitation is a severe susceptibility to oxidation at high temperatures. Exposure to oxygen will cause it to rapidly degrade.
Because of this, pure Mo elements are used almost exclusively in vacuum furnaces or environments with a protective, inert atmosphere (like argon or nitrogen). Their maximum practical operating temperature is typically around 1900°C (3452°F).
Molybdenum Disilicide (MoSi₂) Elements
Molybdenum Disilicide is an advanced ceramic composite designed to overcome the oxidation problem of pure Molybdenum. These elements are workhorses in modern high-temperature electric furnaces.
The defining feature of MoSi₂ is its ability to form a protective surface layer of silica (SiO₂) glass when heated in an oxidizing atmosphere. This thin layer is self-healing and prevents the underlying material from burning away.
This protective mechanism allows MoSi₂ elements to operate reliably at very high temperatures, typically up to 1800°C (3272°F), directly in air.
They are widely used for processes like sintering, annealing, glass manufacturing, and firing ceramics in laboratory and industrial settings.
Core Properties and Performance
Understanding the specific properties of each material is key to selecting the right one for your process.
Electrical and Physical Properties
Molybdenum has good electrical conductivity and a resistivity of 5.20 Ω·mm²/m (at 20°C), making it efficient for resistive heating.
It is a dense metal (10.2 g/cm³) and is available in various forms, including wire, rod, strip, and tubes, offering flexibility in furnace design.
Temperature Limitations
While pure Molybdenum has a very high melting point, its practical use is limited to about 1900°C. Beyond this, it can become excessively brittle.
MoSi₂ elements are typically rated for a maximum temperature of 1800°C. They do not suffer from aging and can operate at high power densities right up to their limit.
Understanding the Trade-offs
Choosing a Molybdenum-based heater requires a clear understanding of its limitations. The primary trade-off revolves around the operating atmosphere.
The Atmosphere is Everything
The most critical factor is the presence of oxygen. Using a pure Mo element in an oxygen-rich environment will lead to rapid failure. It is strictly for vacuum or inert gas applications.
Conversely, a MoSi₂ element relies on oxygen to form its protective silica layer. Using it in a low-oxygen or reducing atmosphere at high temperatures can prevent this layer from forming or healing, leading to material degradation.
Brittleness and Handling
Both types of elements can be brittle, especially after thermal cycling. Pure Molybdenum requires careful handling during installation and maintenance to avoid fractures.
MoSi₂ elements are ceramic and are inherently brittle at room temperature, demanding careful installation to prevent mechanical shock or stress.
Making the Right Choice for Your Goal
To select the correct heating element, you must first define your furnace's operating atmosphere and temperature requirements.
- If your primary focus is heating in a vacuum or inert gas atmosphere: Pure Molybdenum (Mo) elements are the standard choice for temperatures up to 1900°C.
- If your primary focus is heating in an air or oxidizing atmosphere: Molybdenum Disilicide (MoSi₂) elements are designed specifically for this purpose, with reliable performance up to 1800°C.
- If your process involves a reducing atmosphere: Neither element may be ideal, and you should carefully consult manufacturer specifications, as MoSi₂ performance can be compromised.
- If you require flexible element shapes: Pure Molybdenum offers more diverse configurations like wire and strip, whereas MoSi₂ elements are typically rigid rods (often U-shaped).
Matching the specific type of Molybdenum element to your furnace environment is the most important step toward achieving reliable high-temperature performance.
Summary Table:
| Property / Aspect | Pure Molybdenum (Mo) | Molybdenum Disilicide (MoSi₂) |
|---|---|---|
| Melting Point | 2620°C | N/A (ceramic composite) |
| Max Operating Temp | 1900°C | 1800°C |
| Atmosphere | Vacuum or inert gases | Oxidizing (e.g., air) |
| Key Limitation | Susceptible to oxidation | Requires oxygen for protection |
| Common Forms | Wire, rod, strip, tubes | Rigid rods (often U-shaped) |
Struggling to select the right heating element for your high-temperature processes? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we tailor our products to meet your unique experimental needs—whether you're working with pure Molybdenum for vacuum environments or MoSi₂ for oxidizing atmospheres. Contact us today to enhance your lab's efficiency and reliability with precision-engineered heating elements!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- What tasks does a high-temperature vacuum sintering furnace perform for PEM magnets? Achieve Peak Density
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- Why is a high vacuum essential for Ti-6Al-4V sintering? Protect Your Alloys from Embrittlement



















