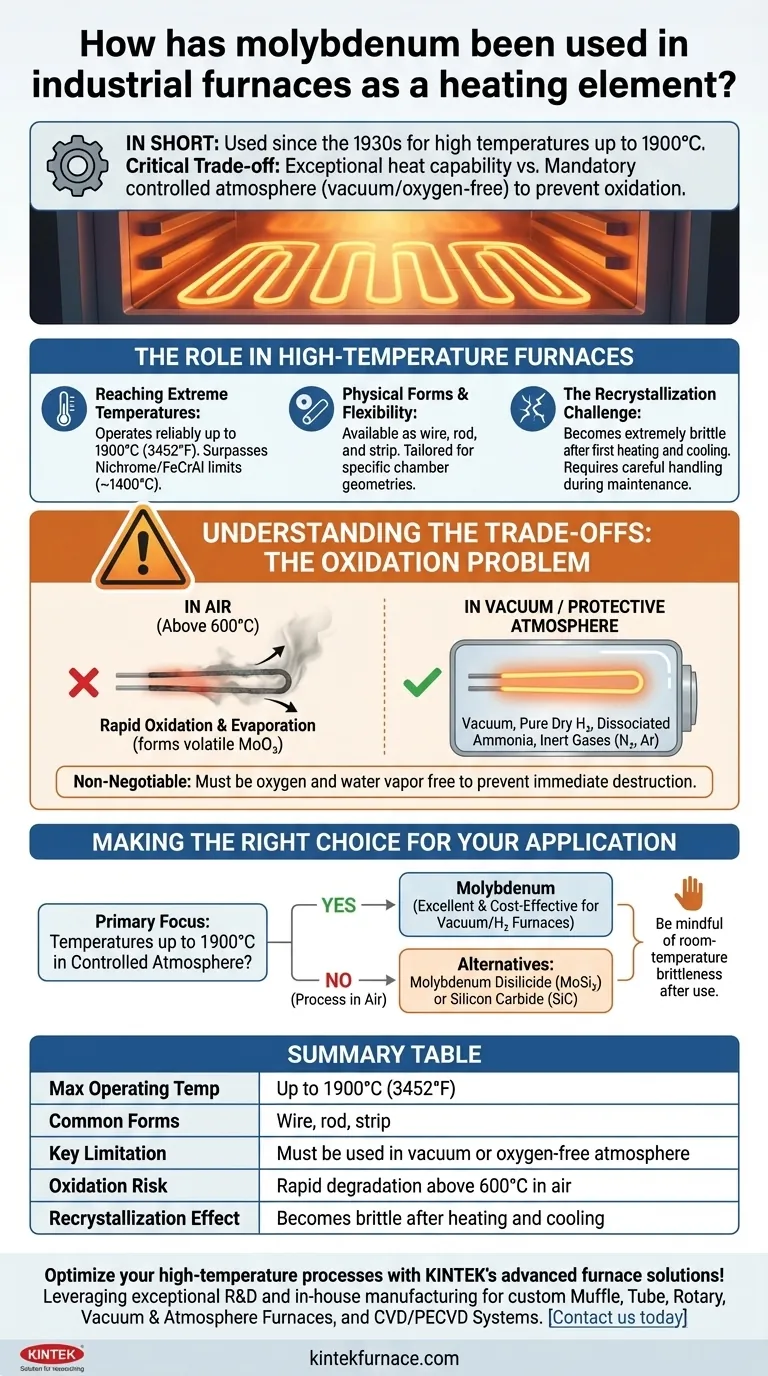
In short, molybdenum has been used as a high-temperature heating element in industrial furnaces since the 1930s for applications requiring temperatures up to 1900°C. It is typically formed into wires or rods, but its primary operational constraint is that it must be used in a vacuum or a protective, oxygen-free atmosphere to prevent rapid degradation.
The core decision to use molybdenum hinges on a critical trade-off: it provides exceptional high-temperature capability at a reasonable cost, but only if you can provide the controlled atmosphere required to protect it from oxidation.
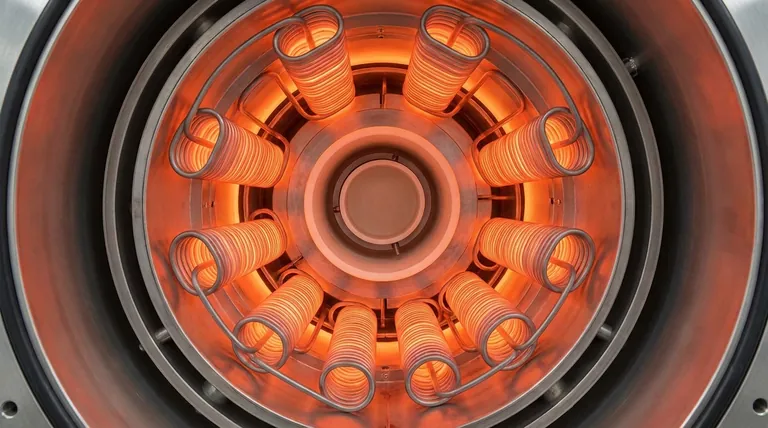
The Role of Molybdenum in High-Temperature Furnaces
Molybdenum occupies a specific niche in furnace design, chosen when standard heating elements cannot meet the required temperature demands. Its properties define both its capabilities and its limitations.
Reaching Extreme Temperatures
The primary reason to select molybdenum is its high melting point and excellent strength at elevated temperatures. This allows it to operate reliably in furnaces at temperatures up to 1900°C (3452°F).
This capability positions it far beyond the limits of more common elements like Nichrome or FeCrAl (Kanthal), which typically fail above 1200-1400°C.
Physical Forms and Design Flexibility
Molybdenum elements are available in various standard configurations, including wire, rod, and strip. This versatility allows furnace designers to create heating arrays tailored to specific chamber geometries and heat distribution requirements.
These elements are often bent into "hairpin" shapes or other configurations to provide uniform heating within the furnace's hot zone.
The Recrystallization Challenge
A critical characteristic to understand is that after being heated to its operating temperature, molybdenum undergoes recrystallization.
Once it cools back to room temperature, the element becomes extremely brittle. This has significant implications for furnace maintenance, as the elements can easily fracture if bumped or handled improperly.
Understanding the Trade-offs: The Oxidation Problem
The single greatest limitation of pure molybdenum is its extreme vulnerability to oxygen at high temperatures. This is not a minor issue; it is a fundamental constraint that dictates the entire furnace design.
Why a Vacuum or Protective Atmosphere is Non-Negotiable
Above approximately 600°C, molybdenum begins to rapidly oxidize in the presence of air. It forms molybdenum trioxide (MoO₃), which is highly volatile at furnace temperatures.
This means the element does not simply form a protective oxide layer—it effectively evaporates, leading to rapid failure of the heating element and contamination of the furnace interior and the product.
Common Protective Atmospheres
To prevent oxidation, molybdenum elements must be operated in a high vacuum or under a protective atmosphere.
Common choices include pure, dry hydrogen, dissociated ammonia, or a mixture of inert gases like nitrogen and argon. The key is the complete absence of oxygen and water vapor.
The Cost and Complexity of Atmosphere Control
This requirement adds significant cost and complexity. The furnace must be vacuum-tight and equipped with expensive vacuum pumps or sophisticated gas management systems to maintain atmospheric integrity.
Any leak or failure in the atmosphere control system can lead to the immediate destruction of the heating elements.
Making the Right Choice for Your Application
Selecting the right heating element requires balancing temperature requirements, atmospheric conditions, and operational constraints.
- If your primary focus is reaching temperatures up to 1900°C in a controlled atmosphere: Molybdenum is an excellent and cost-effective choice for vacuum or hydrogen furnaces.
- If your process must run in an air atmosphere: Pure molybdenum is unsuitable; you must consider alternatives like Molybdenum Disilicide (MoSi₂) or Silicon Carbide (SiC) elements.
- If your application involves frequent maintenance or movement of furnace internals: Be mindful of molybdenum's room-temperature brittleness after its first use and design procedures for careful handling.
Ultimately, understanding molybdenum's relationship with the furnace atmosphere is the key to leveraging its high-temperature capabilities effectively.
Summary Table:
| Feature | Details |
|---|---|
| Max Operating Temperature | Up to 1900°C (3452°F) |
| Common Forms | Wire, rod, strip |
| Key Limitation | Must be used in vacuum or oxygen-free atmosphere |
| Oxidation Risk | Rapid degradation above 600°C in air |
| Recrystallization Effect | Becomes brittle after heating and cooling |
Optimize your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with custom high-temperature furnaces, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs. Contact us today to discuss how our expertise can enhance your efficiency and results!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the purpose of a 1400°C heat treatment for porous tungsten? Essential Steps for Structural Reinforcement
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering



















