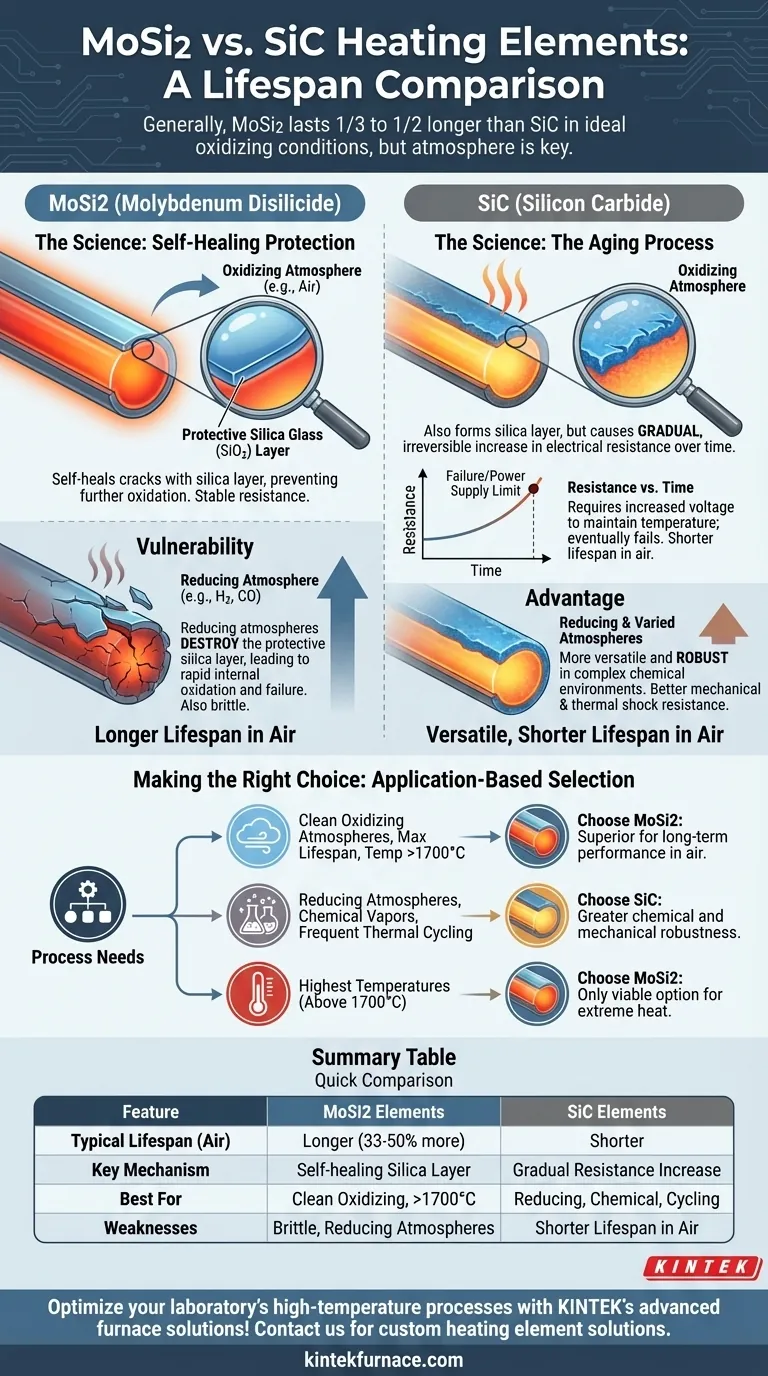
In most high-temperature applications, Molybdenum Disilicide (MoSi2) heating elements offer a significantly longer operational lifespan than Silicon Carbide (SiC) elements. Under ideal conditions, it is common for MoSi2 elements to last one-third to one-half longer than their SiC counterparts.
While MoSi2 elements generally have a longer lifespan, this advantage is critically dependent on the operating atmosphere. The choice between the two is less about a fixed number and more about understanding how the chemistry of your specific process affects the material science of each element.

The Science Behind Element Longevity
To understand the lifespan difference, we must look at how each material behaves at extreme temperatures. Their failure modes are fundamentally different.
How MoSi2 Protects Itself
MoSi2 elements achieve their long life through a process of self-healing. When heated in an oxidizing atmosphere (like air), a thin, protective layer of silica glass (SiO2) forms on the element's surface.
This silica layer is non-porous and prevents further oxidation of the underlying MoSi2 material. If a crack or flaw develops in this layer, the exposed material simply re-oxidizes, effectively "healing" the shield.
The Aging Process of Silicon Carbide
Silicon Carbide elements also oxidize in air, forming a similar silica layer. However, this process in SiC causes the element's electrical resistance to increase slowly and irreversibly over time.
This "aging" means the power supply must be adjusted periodically to deliver more voltage to maintain the desired temperature. Eventually, the resistance becomes too high for the power supply to compensate, or the element fails, defining the end of its useful life.
Understanding the Trade-offs and Vulnerabilities
A longer lifespan for MoSi2 is not guaranteed. Its primary strength is also the source of its greatest weakness.
MoSi2's Critical Weakness: Reducing Atmospheres
The protective silica layer that gives MoSi2 its longevity can be destroyed by certain environments. Reducing atmospheres, which contain gases like hydrogen, carbon monoxide, or dissociated ammonia, will chemically attack and remove the silica layer.
Without this protective barrier, the core MoSi2 material is exposed to rapid internal oxidation and degradation. This can lead to catastrophic failure in a fraction of the time the element would last in clean air.
SiC's Advantage: Atmospheric Versatility
Silicon Carbide is far more robust against varied chemical environments. It performs reliably in oxidizing, neutral, and reducing atmospheres, making it a more versatile and forgiving choice for processes with complex or fluctuating chemistry.
Mechanical and Thermal Shock
MoSi2 elements are notoriously brittle at room temperature and must be handled with extreme care during installation. While SiC is also a ceramic, it is generally more robust and less susceptible to mechanical fracture from handling or thermal shock during rapid temperature cycling.
Making the Right Choice for Your Application
Selecting the correct heating element requires matching the material's properties to your specific operational environment and goals.
- If your primary focus is maximum lifespan in a clean, oxidizing atmosphere (air): MoSi2 is the superior choice due to its self-healing protective layer and stable electrical resistance.
- If your process involves reducing atmospheres, chemical vapors, or frequent thermal cycling: Silicon Carbide offers greater chemical robustness and reliability, making it the safer and more practical option.
- If your priority is reaching the highest possible temperatures (above 1700°C): MoSi2 is often the only viable choice, as it can operate at higher temperatures than standard SiC elements.
By understanding how the atmospheric chemistry of your process interacts with the element, you can confidently select the material that will deliver the best long-term performance and value.
Summary Table:
| Feature | MoSi2 Heating Elements | SiC Heating Elements |
|---|---|---|
| Typical Lifespan | Longer (1/3 to 1/2 longer than SiC in ideal conditions) | Shorter, but more versatile |
| Key Mechanism | Self-healing silica layer in oxidizing atmospheres | Gradual resistance increase over time |
| Best For | Clean oxidizing atmospheres, high temperatures (>1700°C) | Reducing atmospheres, chemical robustness, thermal cycling |
| Weaknesses | Brittle, vulnerable to reducing atmospheres | Shorter lifespan in oxidizing conditions |
Optimize your laboratory's high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems tailored to your unique needs. Our deep customization capabilities ensure precise performance for diverse applications. Contact us today to discuss how our heating elements can extend lifespan and enhance efficiency in your specific environment!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- What makes silicon carbide heating elements resistant to chemical corrosion? Discover the Protective Oxide Layer
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- What is the maximum temperature silicon carbide heating elements can withstand? Key Factors for Longevity and Performance



















