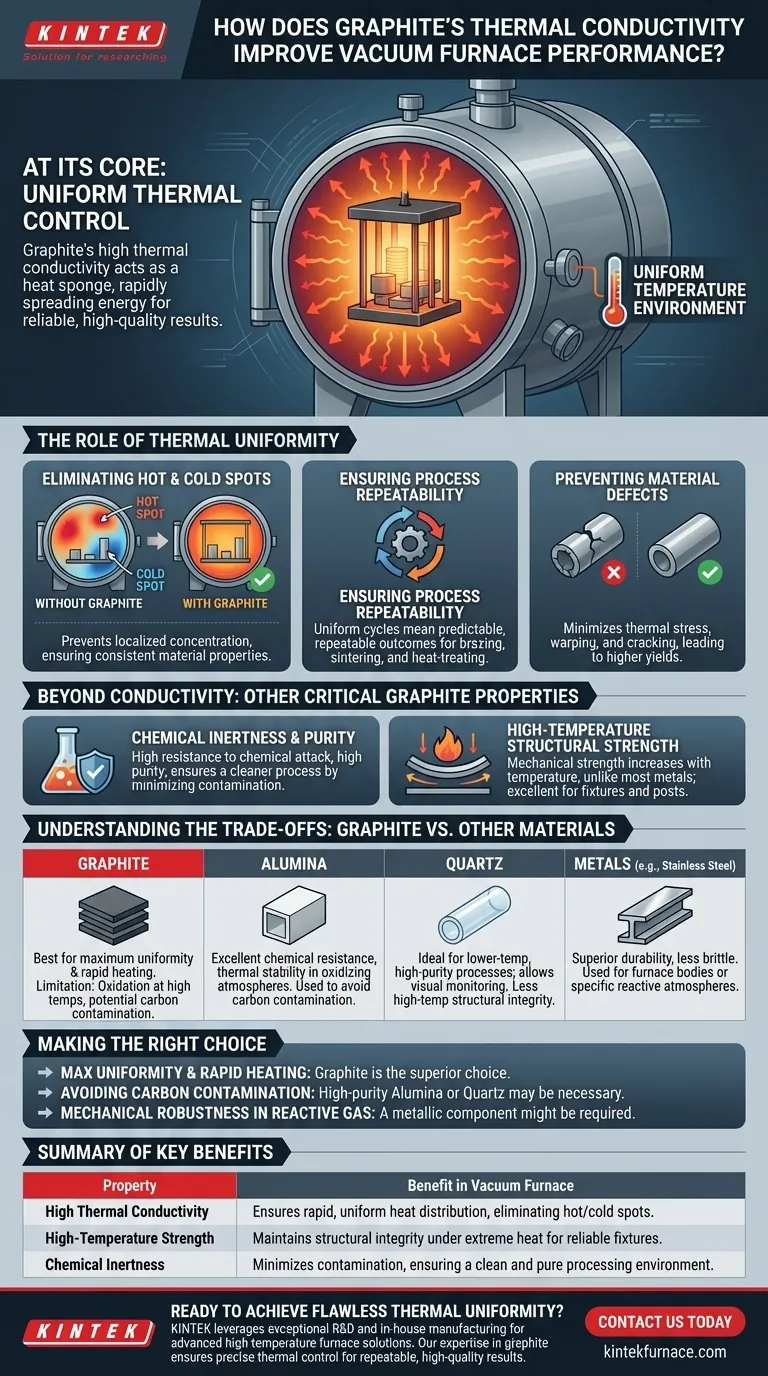
At its core, graphite's high thermal conductivity dramatically improves vacuum furnace performance by creating an exceptionally uniform temperature environment. This ability to spread heat quickly and evenly prevents hot spots, ensuring that every part of the workpiece experiences the same thermal conditions, which is the foundation for repeatable, high-quality results.
The critical insight is that thermal conductivity in a vacuum furnace isn't just about reaching a target temperature; it's about achieving thermal control. Graphite excels at rapidly distributing energy, turning the entire hot zone into a stable, uniform, and predictable processing environment.

The Role of Thermal Uniformity in Vacuum Processing
A vacuum furnace's primary job is to execute a precise thermal cycle. Any deviation in temperature across the workload can compromise the entire process. Graphite's material properties are uniquely suited to prevent this.
Eliminating Hot and Cold Spots
Graphite's high thermal conductivity allows it to act like a heat sponge, rapidly absorbing and distributing thermal energy from the heating elements. This prevents localized heat concentration, ensuring no single area becomes significantly hotter or colder than the setpoint.
Without this uniformity, different parts of a component could undergo different metallurgical transformations, leading to inconsistent material properties.
Ensuring Process Repeatability
When the temperature field is uniform from cycle to cycle, the process becomes highly predictable and repeatable. This is crucial in manufacturing environments where consistency is paramount.
If you can guarantee the same thermal conditions every time, you can guarantee the same outcome, whether you are brazing, sintering, or heat-treating sensitive components.
Preventing Material Defects
Non-uniform heating is a primary cause of material defects. Temperature gradients can induce thermal stress, leading to warping or cracking.
Likewise, cold spots can result in incomplete sintering or failed braze joints. By ensuring the entire workload is at a consistent temperature, graphite directly contributes to higher yields and lower scrap rates.
Beyond Conductivity: Other Critical Graphite Properties
While thermal conductivity is key, other properties of graphite make it an ideal material for the demanding vacuum furnace environment.
Chemical Inertness and Purity
Graphite demonstrates high resistance to chemical attack and can be sourced in very high purities. This ensures a cleaner process by minimizing the risk of contamination from the furnace components themselves.
In a vacuum, where outgassing can ruin a process, using a stable and inert material like graphite is a significant advantage.
High-Temperature Structural Strength
Unlike most metals, which weaken as they heat up, graphite's mechanical strength increases with temperature. This makes it an excellent structural material for furnace fixtures, posts, and heating elements that must bear loads at extreme temperatures.
Understanding the Trade-offs: Graphite vs. Other Materials
Graphite is not the only material used in furnace construction, and choosing the right one depends on the specific application.
Graphite's Limitations
The primary drawback of graphite is its susceptibility to oxidation at high temperatures if the vacuum is compromised or if oxygen is present. For certain highly sensitive applications, it can also be a source of carbon contamination.
When to Consider Alumina
Alumina (an advanced ceramic) offers excellent chemical resistance and thermal stability, particularly in oxidizing atmospheres where graphite cannot be used. It is often chosen for processes where any potential for carbon contamination is unacceptable.
When to Consider Quartz
Quartz tubes are ideal for lower-temperature, high-purity processes. Their transparency also allows for direct visual monitoring, but they lack the high-temperature structural integrity of graphite or alumina.
When to Consider Metals
Specialty metals like stainless steel or molybdenum offer superior durability and are less brittle than graphite. They are often used for furnace bodies or for processes involving specific reactive atmospheres where a metallic tube is required.
Making the Right Choice for Your Application
Selecting the right material requires balancing your process needs with the properties of each option.
- If your primary focus is maximum temperature uniformity and rapid heating: Graphite is almost always the superior choice due to its unmatched thermal conductivity.
- If your primary focus is avoiding carbon contamination in an ultra-pure process: High-purity alumina or quartz may be necessary, accepting their different thermal behaviors.
- If your primary focus is mechanical robustness in a specific reactive gas environment: A metallic furnace component, like one made from stainless steel, might be required.
Ultimately, understanding the interplay between a material's properties and your specific process goals is the key to mastering vacuum furnace performance.
Summary Table:
| Property | Benefit in Vacuum Furnace |
|---|---|
| High Thermal Conductivity | Ensures rapid, uniform heat distribution, eliminating hot/cold spots. |
| High-Temperature Strength | Maintains structural integrity under extreme heat for reliable fixtures. |
| Chemical Inertness | Minimizes contamination, ensuring a clean and pure processing environment. |
Ready to achieve flawless thermal uniformity in your vacuum processes?
At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your exact needs. Our expertise in materials like graphite ensures your furnace delivers the precise thermal control required for repeatable, high-quality results in brazing, sintering, and heat-treating.
Contact us today to discuss how our deep customization capabilities can optimize your vacuum furnace performance. Let's build a solution that guarantees your success.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- What is the primary application of vacuum heat treating furnaces in aerospace? Enhance Component Performance with Precision
- What is the primary function of a vacuum graphite furnace? Achieve Extreme-Temperature Material Purity
- Why are vacuum furnaces used for the re-quenching of samples after a boriding treatment? Master Core Toughness



















