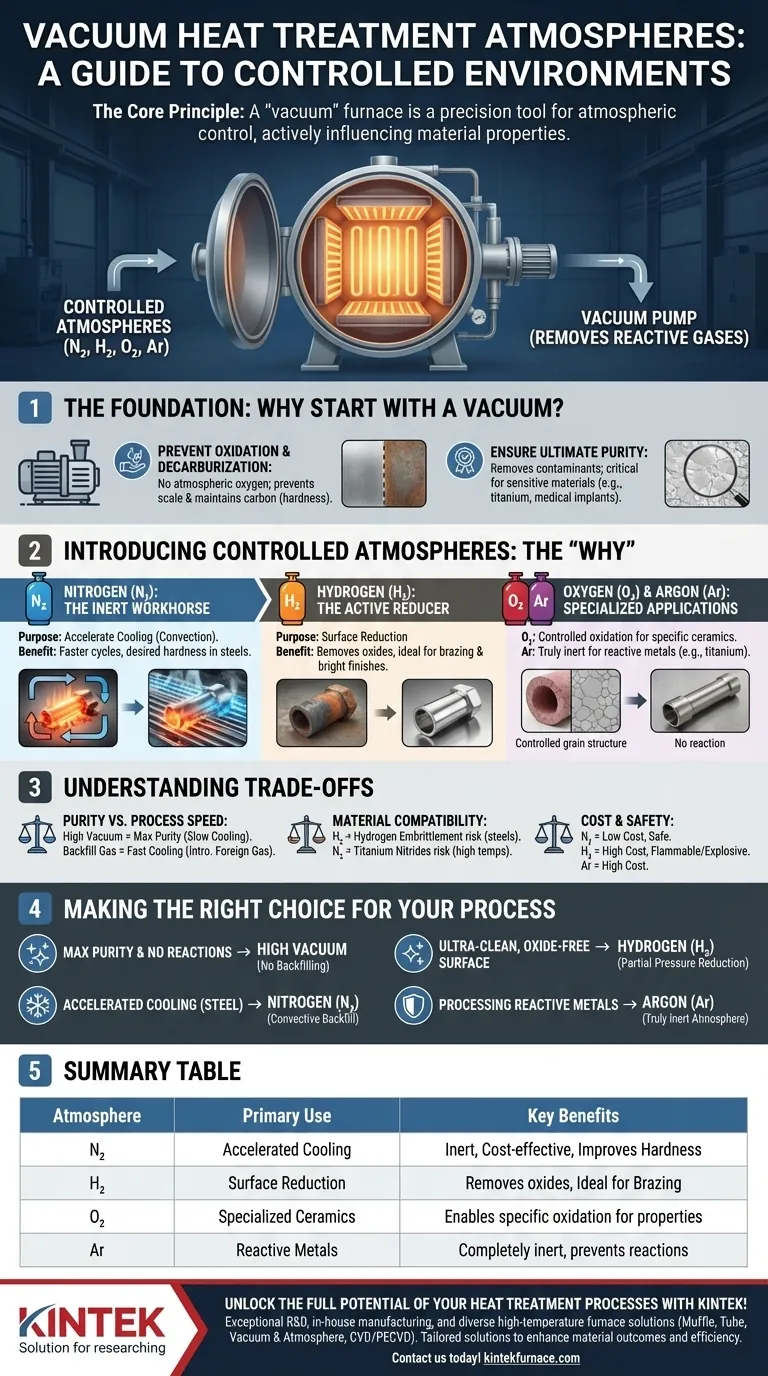
In practice, vacuum heat treatment furnaces primarily operate in a high-vacuum environment, but they can also utilize controlled atmospheres of Nitrogen (N₂), Hydrogen (H₂), and in specialized cases, Oxygen (O₂) or Argon (Ar). The choice of gas is not arbitrary; it is a deliberate decision made to achieve specific metallurgical or ceramic properties that a pure vacuum cannot provide on its own.
The core principle to understand is that a "vacuum" furnace is a tool for atmospheric control. While its primary function is to remove reactive gases, its advanced capability lies in the precise, intentional introduction of a specific atmosphere to actively influence the material's surface chemistry and cooling cycle.

The Foundation: Why Start with a Vacuum?
Before introducing any gas, the furnace chamber is evacuated to create a high vacuum. This initial step is fundamental to the entire process for several critical reasons.
To Prevent Oxidation and Decarburization
The primary purpose of the vacuum is to remove atmospheric oxygen and other reactive gases. This prevents the formation of oxide layers (scale) on the material's surface during heating, preserving its finish and integrity. It also stops decarburization—the loss of carbon from the surface of steel—which maintains the material's designed hardness and strength.
To Ensure Ultimate Purity
By removing atmospheric contaminants, a vacuum provides the cleanest possible environment for heat treatment. This is essential for sensitive materials like titanium alloys, high-temperature superalloys, and medical implants, where even minor surface contamination can lead to component failure.
Introducing Controlled Atmospheres: The "Why"
Once a clean vacuum is established, a specific gas can be introduced at a controlled pressure (a process known as "partial pressure backfilling"). Each gas serves a distinct purpose.
Nitrogen (N₂): The Inert Workhorse
Nitrogen is the most common gas used for backfilling. It is largely inert, meaning it does not readily react with most metals at typical heat treatment temperatures.
Its main purpose is to accelerate cooling. In a pure vacuum, heat can only dissipate through radiation, which is slow. By introducing nitrogen, the furnace can use convection (gas circulation) to cool the parts much faster, which is critical for achieving the desired hardness and microstructure in many steels.
Hydrogen (H₂): The Active Reducer
Hydrogen is an active, not inert, gas. It acts as a powerful reducing agent, meaning it actively strips oxygen from metal oxides.
This is highly useful for processes like brazing, where surfaces must be perfectly clean for the braze alloy to flow and bond properly. It is also used in sintering and for creating bright, oxide-free finishes on materials like stainless steel. Some advanced ceramics are also sintered in a hydrogen atmosphere to achieve specific properties.
Oxygen (O₂) and Other Gases: Specialized Applications
While it seems counterintuitive, a controlled partial pressure of oxygen is sometimes used for firing specific types of advanced ceramics, such as ferroelectric or transparent alumina ceramics. In these niche applications, a precise level of oxidation is required to achieve the desired final chemical structure.
Argon (Ar) is another inert gas, similar to nitrogen, but more expensive. It is used when nitrogen might react with the workpiece material, such as with certain titanium or refractory metals, providing a completely non-reactive environment for both heating and cooling.
Understanding the Trade-offs
Choosing an atmosphere involves balancing process goals with practical constraints. Simply using a high vacuum is not always the optimal or most efficient solution.
Purity vs. Process Speed
A deep vacuum offers the highest purity but suffers from very slow cooling rates. Introducing a backfill gas like nitrogen dramatically speeds up the cooling cycle, increasing throughput, but it introduces a foreign gas into the environment. The choice depends on whether cycle time or absolute purity is the priority.
Material Compatibility
The choice of atmosphere is dictated by the material being processed. Introducing hydrogen into certain steels, for instance, can cause hydrogen embrittlement, a phenomenon that severely reduces the material's ductility and can lead to catastrophic failure. Nitrogen can react with titanium at high temperatures to form titanium nitrides, which may or may not be desirable.
Cost and Safety Implications
Nitrogen is abundant and relatively inexpensive. Argon is significantly more expensive and reserved for applications where nitrogen is unsuitable. Hydrogen is not only costly but also highly flammable and explosive, requiring specialized furnace safety systems and handling protocols.
Making the Right Choice for Your Process
Your selection of a furnace atmosphere should be driven directly by the intended outcome for your material.
- If your primary focus is maximum purity and preventing all surface reactions: Use a high vacuum environment with no backfilling.
- If your primary focus is accelerated cooling for steel hardening: Use Nitrogen (N₂) as a convective backfill gas during the quench cycle.
- If your primary focus is creating an ultra-clean, oxide-free surface for brazing or annealing: Use a partial pressure of Hydrogen (H₂) to actively reduce surface oxides.
- If your primary focus is processing highly reactive metals like titanium: Use Argon (Ar) as a truly inert atmosphere to prevent unwanted reactions.
Ultimately, mastering vacuum heat treatment means viewing the atmosphere not as an absence of something, but as the most critical, controllable parameter in your process.
Summary Table:
| Atmosphere | Primary Use | Key Benefits |
|---|---|---|
| Nitrogen (N₂) | Accelerated cooling | Inert, cost-effective, improves hardness |
| Hydrogen (H₂) | Surface reduction | Removes oxides, ideal for brazing |
| Oxygen (O₂) | Specialized ceramics | Enables specific oxidation for properties |
| Argon (Ar) | Reactive metals | Completely inert, prevents reactions |
Unlock the full potential of your heat treatment processes with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by strong deep customization capabilities to precisely meet your unique experimental requirements. Contact us today to discuss how our tailored solutions can enhance your material outcomes and efficiency!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the proper procedures for handling the furnace door and samples in a vacuum furnace? Ensure Process Integrity & Safety
- What are the general operational features of a vacuum furnace? Achieve Superior Material Purity & Precision
- What are the functions of a high-vacuum furnace for CoReCr alloys? Achieve Microstructural Precision and Phase Stability
- How does a vacuum heat treatment furnace influence Ti-6Al-4V microstructure? Optimize Ductility and Fatigue Resistance
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion



















