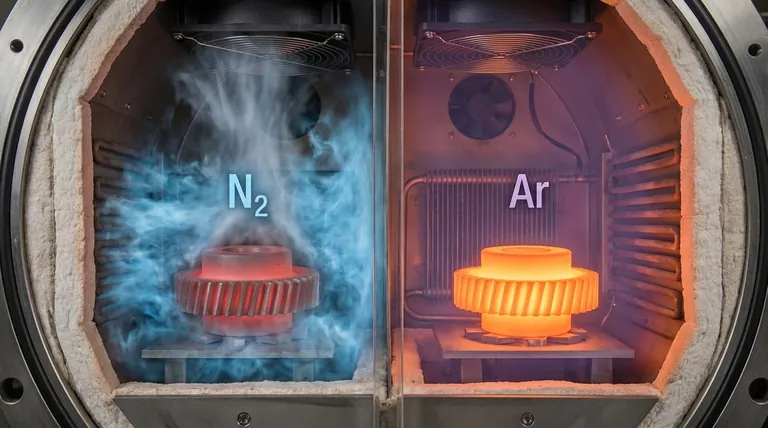
In a direct comparison, nitrogen provides a faster cooling rate in vacuum furnaces than argon. This is primarily because nitrogen has better thermal conductivity, allowing it to absorb and transfer heat away from the workpiece more efficiently. However, the choice is not simply about speed; it involves critical trade-offs in process compatibility, material integrity, and operational safety.
While nitrogen is the more efficient cooling agent, argon's complete inertness and higher density make it essential for sensitive materials. Your choice depends on whether your priority is maximizing cooling speed or guaranteeing the chemical purity and final properties of your part.
The Physics of Furnace Cooling: Why Gas Choice Matters
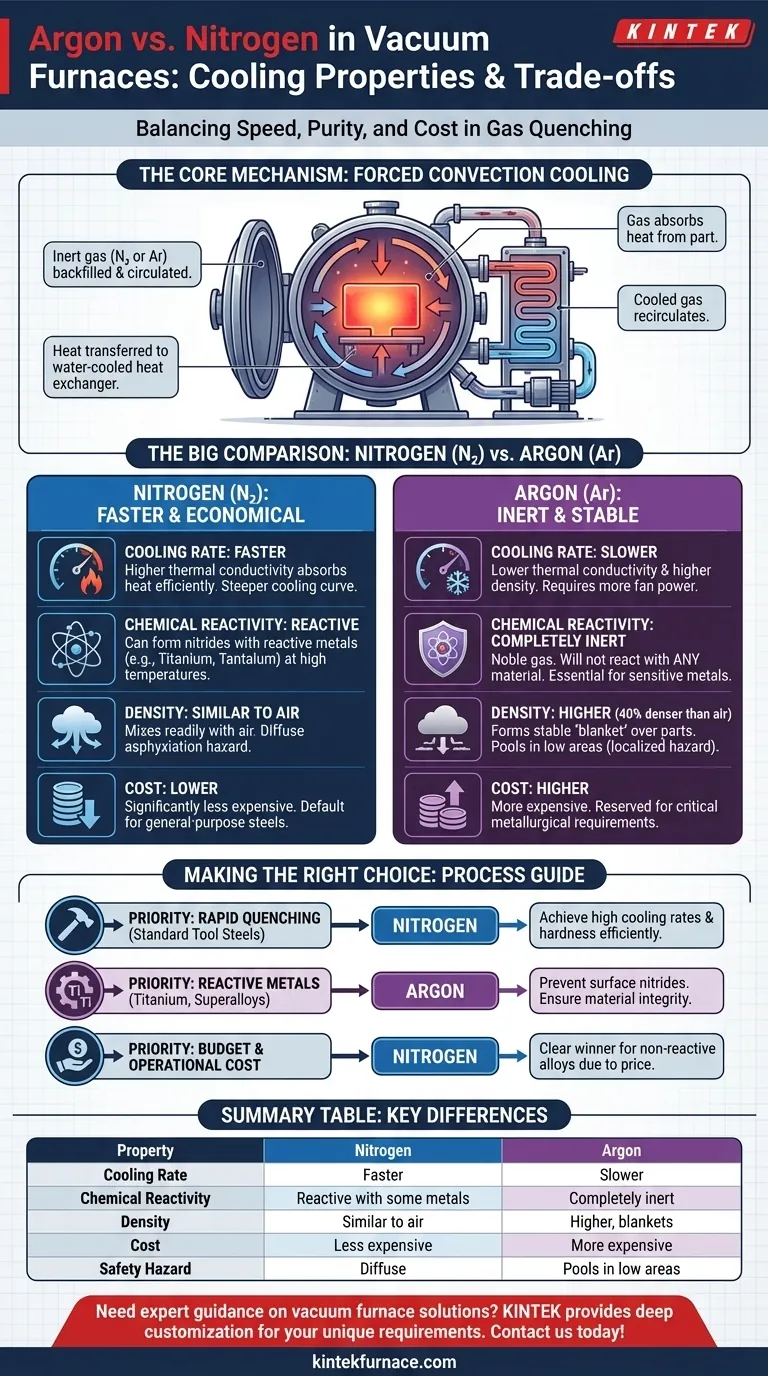
To understand the difference, we must first look at the mechanism of gas quenching in a vacuum furnace. The process relies on forced convection to remove heat from the part.
The Core Mechanism: Convective Heat Transfer
After the heating cycle is complete, the vacuum chamber is backfilled with an inert gas like nitrogen or argon to a positive pressure, often two times atmospheric pressure or more.
A powerful fan circulates this gas at high velocity. The gas absorbs heat from the hot workpiece, carries it to a heat exchanger (typically water-cooled), and then recirculates back into the hot zone to absorb more heat.
Nitrogen: The Faster Cooling Agent
Nitrogen is the superior gas for rapid cooling. Its molecular structure gives it a higher specific heat and thermal conductivity compared to argon.
This means that for every cubic foot of gas circulated, nitrogen can absorb and transport more thermal energy away from the part than argon can. This results in a steeper cooling curve and shorter cycle times.
Argon: The Slower, More Stable Agent
Samples cool more slowly in argon. This is due to two main properties: its lower thermal conductivity and its higher density.
Because argon is less effective at transferring heat, the quenching process is inherently slower. Additionally, its high density requires more energy from the circulation fan to achieve the same gas velocity as nitrogen, which can further limit the maximum cooling rate depending on the furnace design.
Beyond Cooling Speed: Critical Process Factors
The optimal gas is not always the one that cools the fastest. The metallurgical requirements of the material being processed are often the deciding factor.
Chemical Reactivity: When Purity is Paramount
This is the most critical distinction. While both gases are considered "inert," nitrogen can react with certain elements at the high temperatures found in a vacuum furnace.
For example, nitrogen will react with titanium, tantalum, and some stainless steels to form nitrides on the surface of the part. This can alter the material's properties and is usually undesirable.
Argon, as a noble gas, is completely inert under all furnace conditions. It will not react with any material, making it the only safe choice for processing highly reactive metals.
Gas Density and Blanketing
Argon is approximately 40% denser than air, while nitrogen's density is very similar to air. This difference has practical implications.
Argon's high density allows it to form a stable "blanket" over parts, effectively shielding them from trace contaminants with minimal gas flow. This can be an advantage in static cooling or low-flow applications.
The Influence of Furnace Design
The choice of gas is only one part of the equation. The efficiency of a furnace's quenching system—including the power of its circulation fan, the efficiency of its heat exchanger, and the design of its gas nozzles—plays an enormous role in the final cooling rate. A well-designed system running argon can outperform a poorly designed one running nitrogen.
Understanding the Trade-offs: Safety and Cost
Practical considerations like operator safety and operational cost are just as important as the technical performance of the gas.
The Asphyxiation Hazard: A Key Safety Distinction
Both gases are asphyxiants, meaning they can displace oxygen in an enclosed space and are fatal if inhaled. However, their densities create different types of hazards in the event of a leak.
Being denser than air, argon will pool in low-lying areas like pits or basements, leaving breathable air above. Nitrogen will mix readily with the air, creating a diffuse hazard throughout a room that is harder to detect without monitors. Both require proper ventilation and oxygen monitoring.
Cost-Effectiveness
There is a significant cost difference between the two gases. Nitrogen is far less expensive than argon.
For this reason, nitrogen is the default choice for general-purpose heat treating of common steels and other non-reactive alloys. Argon is typically reserved for processes where its chemical inertness is a strict metallurgical requirement.
Making the Right Choice for Your Process
Your selection of a quenching gas must be a deliberate decision based on your material, your process goals, and your operational constraints.
- If your primary focus is rapid quenching of standard tool steels: Nitrogen is the more effective and economical choice for achieving high cooling rates and desired hardness.
- If you are processing highly reactive metals like titanium or certain superalloys: Argon is mandatory to prevent the formation of unwanted surface nitrides and ensure material integrity.
- If your goal is controlled, slow cooling for annealing or stress relief: Either gas can be used, but argon's 'blanketing' effect may offer better stability with lower gas flow if the material is sensitive.
- If budget and operational cost are the main drivers: Nitrogen is the clear winner due to its lower price point, provided it is chemically compatible with your material.
Ultimately, selecting the right gas is about balancing the thermal demands of your process with the chemical constraints of your material.
Summary Table:
| Property | Nitrogen | Argon |
|---|---|---|
| Cooling Rate | Faster due to higher thermal conductivity | Slower due to lower thermal conductivity and higher density |
| Chemical Reactivity | Can react with reactive metals (e.g., titanium) | Completely inert, safe for all materials |
| Density | Similar to air, mixes easily | Higher, forms stable blanket |
| Cost | Less expensive | More expensive |
| Safety Hazard | Diffuse asphyxiation risk | Pools in low areas, localized risk |
Need expert guidance on selecting the right cooling gas for your vacuum furnace? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements, whether you're processing reactive metals or optimizing for speed and cost. Contact us today to discuss how our tailored solutions can enhance your lab's efficiency and material outcomes!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing



















