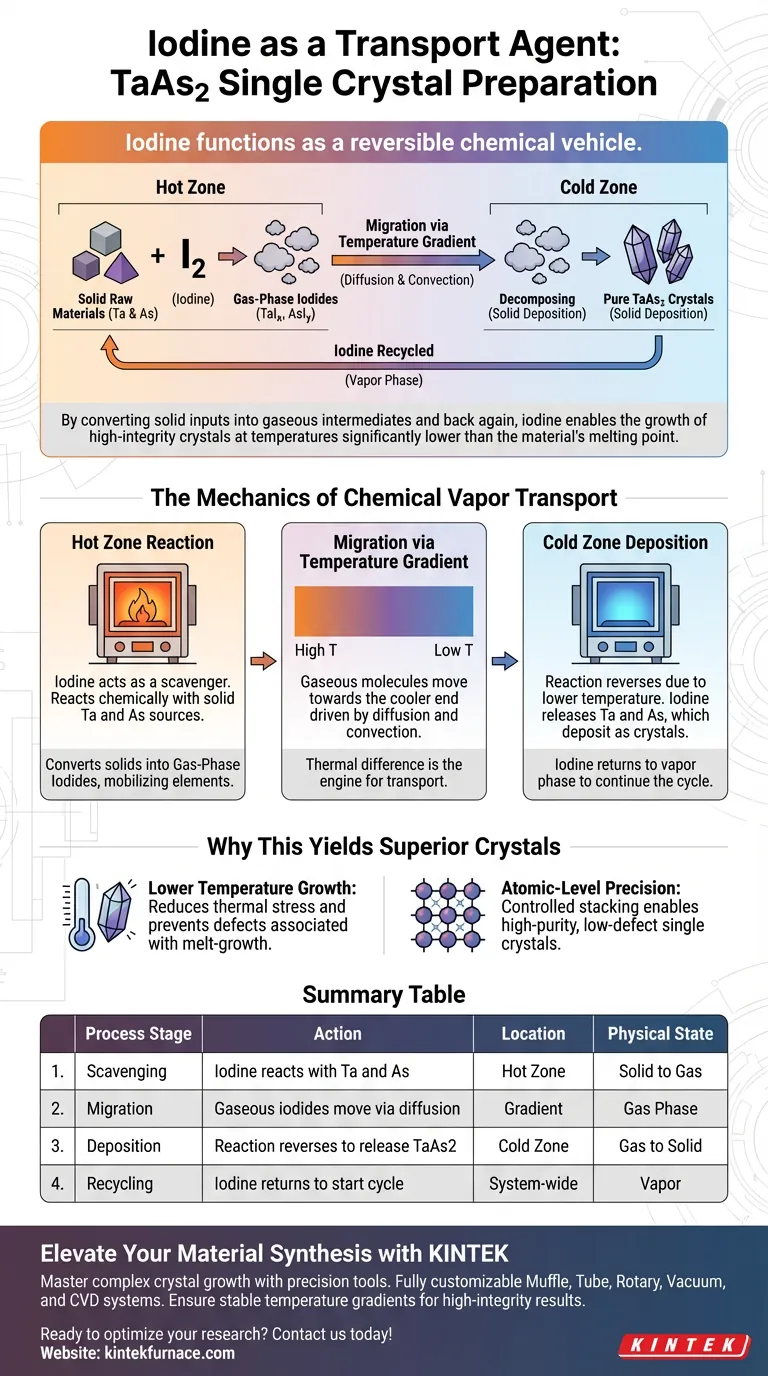
Iodine functions as a reversible chemical vehicle. In the preparation of TaAs2 single crystals, iodine reacts with solid raw materials at a high-temperature zone to form volatile gas-phase iodides. These gases migrate to a lower-temperature zone, where the reaction reverses to deposit pure TaAs2 crystals, effectively transporting the material without melting it.
By converting solid inputs into gaseous intermediates and back again, iodine enables the growth of high-integrity crystals at temperatures significantly lower than the material's melting point.
The Mechanics of Chemical Vapor Transport
To understand how high-quality TaAs2 crystals are formed, one must look at the specific thermodynamic role iodine plays within the sealed reaction environment.
The Hot Zone Reaction
At the "hot end" of the reaction vessel, iodine acts as a scavenger. It reacts chemically with the solid Tantalum (Ta) and Arsenic (As) sources.
This reaction converts the solid raw materials into gas-phase iodides. This phase transition is critical because it mobilizes elements that would otherwise remain stationary solids.
Migration via Temperature Gradient
Once in the gas phase, the material is no longer static. Driven by diffusion and convection, these gaseous molecules move toward the cooler end of the vessel.
The temperature gradient between the hot and cold zones acts as the engine for this transport. Without this specific thermal difference, the net movement of material would not occur.
The Cold Zone Deposition
Upon reaching the "cold end," the thermodynamic balance shifts. The lower temperature causes the gas-phase iodides to become unstable.
Consequently, the reaction reverses: the iodine releases the Tantalum and Arsenic, which deposit as solid TaAs2 crystals. The iodine is released back into the vapor phase to return to the hot zone, continuing the cycle.
Why This Yields Superior Crystals
The use of iodine is not just about moving material; it is about controlling how the material re-solidifies.
Lower Temperature Growth
A primary advantage of this method is thermal management. As noted in the primary reference, this mechanism allows for crystal growth at temperatures significantly lower than the melting point of TaAs2.
Growing below the melting point reduces thermal stress and prevents the formation of defects often associated with melt-growth techniques.
Atomic-Level Precision
The transition from gas to solid facilitates a highly ordered structure. As the gas-phase components decompose at the cold end, they undergo atomic-level rearrangement.
This controlled stacking of atoms enables the formation of high-purity, low-defect single crystals with high structural integrity.
Understanding the Trade-offs
While iodine transport is effective, it introduces specific variables that must be strictly managed to ensure success.
Reliance on Precise Gradients
The process is entirely dependent on the stability of the temperature gradient. If the temperature difference between the hot and cold zones fluctuates, the transport rate becomes unpredictable.
Complexity of Reaction Kinetics
The formation of gas-phase iodides is a delicate chemical balance. The specific partial pressures of the iodine and the transport species must be optimized to prevent the transport from stalling or occurring too rapidly, which could degrade crystal quality.
Making the Right Choice for Your Goal
When deciding whether to utilize iodine transport for crystal synthesis, consider your specific constraints regarding temperature and quality.
- If your primary focus is Structural Integrity: The gas-phase transport mechanism is ideal because it allows for atomic-level rearrangement, minimizing internal defects.
- If your primary focus is Thermal Constraints: This method is essential if your material has a melting point that is prohibitively high for standard furnaces, as it bypasses the liquid phase entirely.
By leveraging the reversible reactivity of iodine, you gain precise control over the crystallization process, decoupling the growth temperature from the material's melting point.
Summary Table:
| Process Stage | Action | Location | Physical State |
|---|---|---|---|
| Scavenging | Iodine reacts with Ta and As | Hot Zone | Solid to Gas |
| Migration | Gaseous iodides move via diffusion | Gradient | Gas Phase |
| Deposition | Reaction reverses to release TaAs2 | Cold Zone | Gas to Solid |
| Recycling | Iodine returns to start cycle | System-wide | Vapor |
Elevate Your Material Synthesis with KINTEK
Are you looking to master complex crystal growth or high-temperature chemical vapor deposition? KINTEK provides the precision tools you need to succeed. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique laboratory requirements.
Whether you are performing iodine-mediated transport or advanced semiconductor research, our high-temp furnaces ensure the stable temperature gradients essential for high-integrity results.
Ready to optimize your research? Contact us today to find your custom furnace solution!
Visual Guide
References
- Haiyao Hu, Claudia Felser. Multipocket synergy towards high thermoelectric performance in topological semimetal TaAs2. DOI: 10.1038/s41467-024-55490-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano Diamond Coating
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
People Also Ask
- What are the drawbacks of CVD compared to PECVD? Key Limitations for Your Lab
- What is chemical vapor deposition (CVD) and its primary industrial application? Unlock Precision Thin Films for Electronics
- How is CVD utilized in coating applications? Unlock High-Performance Surface Engineering
- What is Chemical Vapor Infiltration (CVI)? Build Dense, High-Performance Composites
- What factors should be considered when choosing a CVD furnace? Key Tips for Optimal Thin-Film Synthesis
- How does Chemical Vapor Deposition (CVD) work? Master Thin Film Fabrication for Superior Materials
- What are the primary functions of a high vacuum pump system within a CVD graphene process? Ensure High-Purity Synthesis
- What is the role of an open-flow cold-wall CVD system in HfO2 preparation? Achieve High Purity & Uniformity



