Metal catalysts act as liquid architects for nanowire structures. In a high-temperature furnace, metal particles (typically gold) absorb Zinc Sulfide (ZnS) vapor to form a liquid alloy droplet. This droplet becomes supersaturated and forces the ZnS to precipitate solely from the bottom, resulting in the continuous, upward growth of a one-dimensional nanowire.
The core function of the metal catalyst is to serve as a localized "trap" for vapors. By converting gas-phase precursors into a liquid alloy and restricting precipitation to a specific interface, the catalyst enforces a strict, unidirectional growth pattern that results in high-aspect-ratio nanowires.

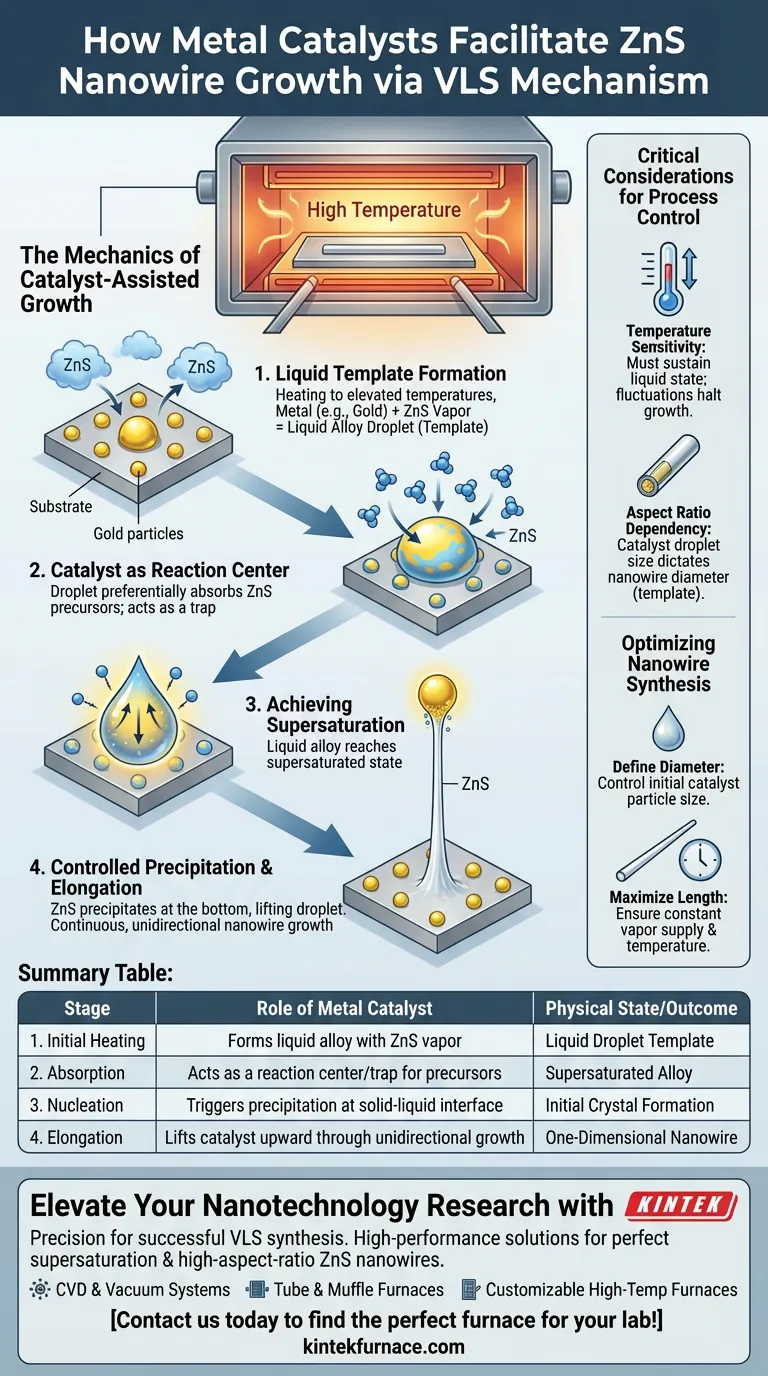
The Mechanics of Catalyst-Assisted Growth
The Vapor-Liquid-Solid (VLS) mechanism is a phase-changing process defined by the distinct role of the catalyst particle.
Formation of the Liquid Template
The process begins with metal catalyst particles, such as gold, deposited on a substrate.
As the furnace reaches elevated temperatures, these solid metal particles interact with ZnS vapors. This interaction creates liquid alloy droplets that sit on the substrate surface, serving as the physical foundation for growth.
The Catalyst as a Reaction Center
Once the liquid droplet forms, it acts as a highly efficient collection site.
The droplet serves as a reaction center that preferentially absorbs gas-phase precursors from the surrounding environment. It captures ZnS vapor much more effectively than the solid substrate itself could.
Achieving Supersaturation
The droplet continues to absorb precursors until it can hold no more.
Eventually, the liquid alloy reaches a supersaturated state. This thermodynamic instability is the trigger that initiates the transition from liquid back to solid.
Controlled Precipitation
To relieve the supersaturation, the ZnS precipitates out of the alloy.
Crucially, this precipitation occurs only at the bottom of the droplet, at the interface between the liquid and the substrate. As the solid material builds up, it lifts the droplet upward, creating a continuous, unidirectional nanowire.
Critical Considerations for Process Control
While the VLS mechanism is powerful, it relies heavily on maintaining specific physical conditions within the furnace.
Temperature Sensitivity
The furnace must maintain elevated temperatures sufficient to sustain the liquid state of the alloy droplet.
If the temperature fluctuates or drops too low, the droplet may solidify prematurely, halting the absorption of vapors and terminating the growth of the nanowire.
Aspect Ratio Dependency
The resulting geometry of the nanowire is directly dictated by the catalyst.
Because the catalyst acts as a physical template, the diameter of the growing wire corresponds to the size of the alloy droplet. This relationship allows for the synthesis of wires with extremely high aspect ratios (long length relative to width).
Optimizing Nanowire Synthesis
To achieve specific results with ZnS nanowires, you must manipulate the catalyst and the environment.
- If your primary focus is defining wire diameter: Control the initial size of the metal catalyst particles deposited on the substrate, as they dictate the droplet size.
- If your primary focus is maximizing length: Ensure the supply of ZnS vapor and the furnace temperature remain constant to maintain the supersaturated state of the droplet over time.
By precisely managing the catalyst particle, you convert a chaotic vapor environment into an orderly, one-dimensional crystalline structure.
Summary Table:
| Stage of VLS Process | Role of Metal Catalyst (e.g., Gold) | Physical State/Outcome |
|---|---|---|
| 1. Initial Heating | Forms liquid alloy with ZnS vapor | Liquid Droplet Template |
| 2. Absorption | Acts as a reaction center/trap for precursors | Supersaturated Alloy |
| 3. Nucleation | Triggers precipitation at the solid-liquid interface | Initial Crystal Formation |
| 4. Elongation | Lifts catalyst upward through unidirectional growth | One-Dimensional Nanowire |
Elevate Your Nanotechnology Research with KINTEK
Precision is the backbone of successful Vapor-Liquid-Solid (VLS) synthesis. To achieve the perfect supersaturation and high-aspect-ratio ZnS nanowires described above, you need unwavering thermal stability.
KINTEK provides high-performance heating solutions backed by expert R&D and manufacturing. Our range includes:
- CVD & Vacuum Systems: Optimized for precise vapor delivery and atmosphere control.
- Tube & Muffle Furnaces: Offering the temperature uniformity essential for catalyst liquid-state maintenance.
- Customizable High-Temp Furnaces: Tailored specifically for unique laboratory or industrial synthesis needs.
Whether you are refining wire diameter or maximizing growth length, KINTEK's equipment ensures your metal catalysts perform as intended. Contact us today to find the perfect furnace for your lab!
Visual Guide
References
- Amartya Chakrabarti, Emily Alessandri. Syntheses, Properties, and Applications of ZnS-Based Nanomaterials. DOI: 10.3390/applnano5030010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is tube CVD? A Guide to High-Purity Thin Film Synthesis
- How is the CVD process environment created? Master Precise Control for Superior Thin Films
- What role does a thermal evaporation coating system play in GeCC synthesis? Precision Seeding for Nanowire Growth
- What are the key characteristics of a CVD coating process? Unlock Superior Adhesion and Complex Coating
- Why is vacuum evaporation equipment necessary for g-C3N4 electronic devices? Achieving Atomic Interface Precision
- Why is hot-wall MOCVD preferred for β-Ga2O3? Boost Crystal Quality with Superior Thermal Control
- What is Chemical Vapor Deposition (CVD) used for? Unlock High-Performance Thin Films for Your Applications
- What plasma methods are used in CVD processes? Discover Low-Temperature Solutions for Sensitive Substrates

