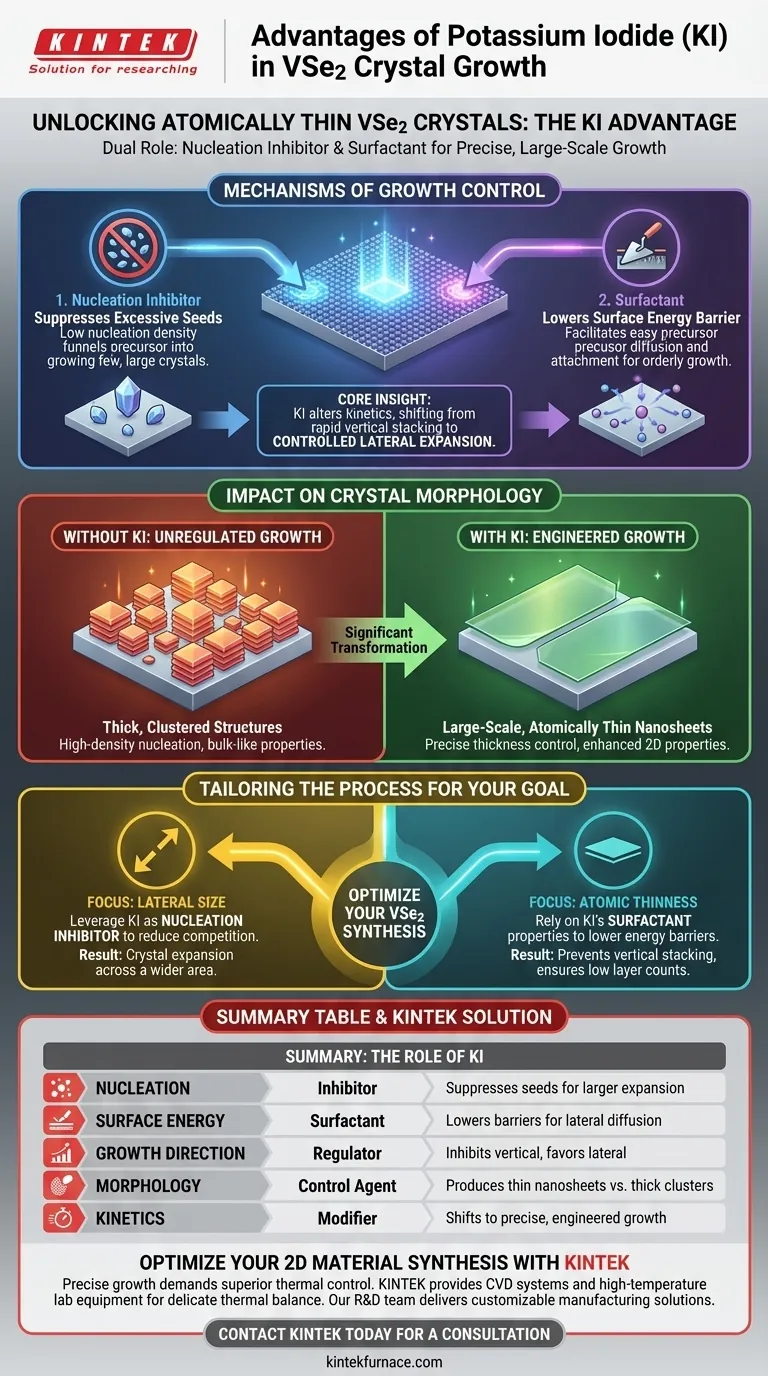
The primary advantage of using alkali metal halides like Potassium Iodide (KI) is their ability to simultaneously act as nucleation inhibitors and surfactants during crystal synthesis. By introducing KI, you can effectively suppress the formation of excessive crystal seeds, ensuring the final VSe2 product grows as large, atomically thin nanosheets rather than thick, clustered structures.
Core Insight: KI fundamentally alters the growth kinetics by lowering the energy barrier on the substrate surface. This regulation shifts the system from rapid, uncontrolled vertical stacking to controlled lateral expansion, enabling the production of large-scale 2D materials with precise thickness.

Mechanisms of Growth Control
The Dual Role of KI
Potassium Iodide operates through two distinct but complementary mechanisms: it functions as a nucleation inhibitor and a surfactant.
This dual functionality addresses the chaotic nature of standard chemical vapor deposition (CVD) growth. Without such an additive, precursor atoms often clump together too rapidly.
Reducing Surface Energy Barriers
As a surfactant, KI modifies the interaction between the growing crystal and the substrate.
The presence of the halide lowers the energy barrier on the growth surface. This thermodynamic change makes it easier for precursor atoms to diffuse across the surface and attach to the edges of existing crystals, promoting orderly growth.
Regulating Nucleation Density
Uncontrolled nucleation results in many small crystals fighting for space.
KI suppresses these excessive nucleation events. By keeping the nucleation density low, the available precursor material is funneled into growing a few large crystals rather than generating thousands of microscopic ones.
Impact on Crystal Morphology
Achieving Large-Scale Lateral Growth
The suppression of competing nuclei allows the remaining crystals to expand outward without obstruction.
This leads to the formation of laterally extended nanosheets. The crystals grow "wide" rather than crowding each other out, resulting in significantly larger surface areas.
Precise Thickness Control
Perhaps the most critical advantage is the ability to maintain extremely low layer counts.
By inhibiting vertical growth mechanisms and promoting surface diffusion, KI ensures the material remains atomically thin. This makes it an essential auxiliary process for synthesizing high-quality 2D materials where thickness determines electronic properties.
Understanding the Critical Balance
The Necessity of Regulation
While KI promotes growth, its primary function is regulation.
The process relies on a delicate balance between inhibiting new seeds and allowing existing ones to grow. If this balance is not maintained via the additive, the system reverts to high-density nucleation.
Consequences of Omitting Additives
Without a growth promoter like KI, the energy barrier on the surface remains high.
This typically results in "unregulated" growth, leading to thicker, bulk-like crystals that lack the unique 2D properties desired in VSe2 applications.
Making the Right Choice for Your Goal
To maximize the quality of your VSe2 crystals, you must tailor the use of KI to your specific structural requirements.
- If your primary focus is Lateral Size: Leverage KI primarily as a nucleation inhibitor to reduce competition, allowing crystals to expand across a wider area.
- If your primary focus is Atomic Thinness: Rely on KI's surfactant properties to lower surface energy barriers, preventing vertical stacking and ensuring low layer counts.
By effectively utilizing Potassium Iodide, you move from random chemical deposition to precise, engineered crystal growth.
Summary Table:
| Feature | Role of KI (Alkali Metal Halide) | Impact on VSe2 Growth |
|---|---|---|
| Nucleation | Inhibitor | Suppresses excessive seeds to allow larger crystal expansion |
| Surface Energy | Surfactant | Lowers energy barriers to promote lateral atom diffusion |
| Growth Direction | Regulator | Inhibits vertical stacking in favor of wide lateral expansion |
| Morphology | Control Agent | Produces large-scale, atomically thin nanosheets vs. thick clusters |
| Kinetics | Modifier | Shifts growth from rapid, uncontrolled to precise, engineered |
Optimize Your 2D Material Synthesis with KINTEK
Precise crystal growth requires more than just the right chemical additives—it demands superior thermal control. KINTEK provides industry-leading CVD systems, vacuum furnaces, and high-temperature lab equipment designed to maintain the delicate thermal balance needed for alkali metal halide-assisted growth.
Whether you are synthesizing VSe2 nanosheets or exploring new 2D materials, our expert R&D team can deliver customizable manufacturing solutions tailored to your unique research needs.
Ready to elevate your material quality? Contact KINTEK today for a consultation and see how our advanced furnace systems can enhance your lab's efficiency.
Visual Guide
References
- Gangtae Jin. Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. DOI: 10.3390/nano15070548
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
People Also Ask
- What are the methods for depositing silicon dioxide using CVD? Optimize Your Semiconductor Process
- What is Chemical Vapor Deposition (CVD) used for? Unlock High-Performance Thin Films for Your Applications
- What are the key differences between PVD and CVD processes? Choose the Right Thin-Film Deposition Method
- What is the function of an infrared pyrometer in β-Ga2O3 growth? Key to Precise MOCVD Temperature Control
- What is the difference between chemical vapor transport and chemical vapor deposition? A Guide to Coating vs. Crystal Growth
- How does graphene skin via FB-CVD improve thermal conductivity? Unlock Advanced Heat Transfer in Composites
- How does a customized hot-wall ALD reactor contribute to 6FDA-TFDB membranes? Enhance Atomic-Level Polymer Modification
- What are the future trends in CVD technology? AI, Sustainability, and Advanced Materials


