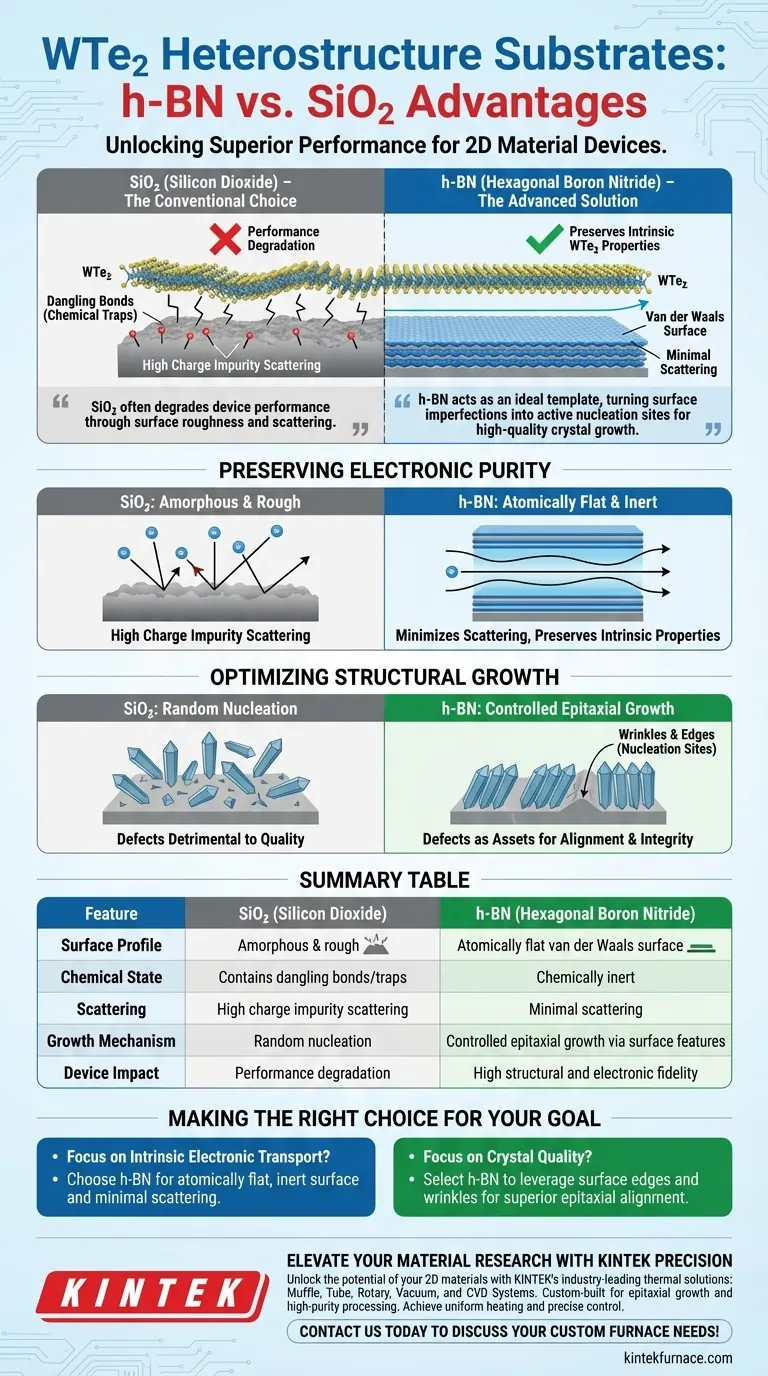
The choice of substrate fundamentally dictates the electronic fidelity of your heterostructure. High-purity hexagonal boron nitride (h-BN) outperforms silicon dioxide (SiO2) by providing an atomically flat, chemically inert surface that drastically reduces charge impurity scattering. Additionally, h-BN uniquely leverages specific surface features to promote epitaxial growth, resulting in superior structural integrity for tungsten ditelluride (WTe2) devices.
While SiO2 often degrades device performance through surface roughness and scattering, h-BN preserves the intrinsic properties of WTe2. It acts as an ideal template, turning surface imperfections into active nucleation sites for high-quality crystal growth.

Preserving Electronic Purity
The Van der Waals Interface
h-BN provides a chemically inert van der Waals surface. Unlike silicon dioxide, it provides an interface free of dangling bonds and chemical traps.
Reduction of Scattering
The atomic flatness of high-purity h-BN significantly minimizes charge impurity scattering. This allows you to preserve and observe the intrinsic electronic properties of the active WTe2 material, which are often masked by the roughness of SiO2.
Optimizing Structural Growth
Defects as Assets
On standard substrates like SiO2, surface defects are usually detrimental to device quality. However, on h-BN, specific surface defect sites—such as wrinkles or edges—serve a functional purpose.
Epitaxial Nucleation
These distinct surface features act as nucleation centers. They actively promote the epitaxial growth of tungsten ditelluride, ensuring the crystal aligns correctly during formation.
Vertical Integrity
This controlled nucleation process facilitates the creation of high-quality vertical heterostructures. The resulting material exhibits superior structural integrity compared to WTe2 grown on amorphous oxide surfaces.
Understanding the Trade-offs
Dependence on Surface Features
The advantage of h-BN relies heavily on the presence and distribution of specific surface features. The growth mechanism utilizes wrinkles and edges as seeding points.
Uniformity Considerations
If the h-BN surface is too perfect or lacks these specific nucleation centers, the epitaxial growth benefits may be diminished. You are trading the random roughness of SiO2 for a reliance on specific, localized structural cues on the h-BN surface.
Making the Right Choice for Your Goal
- If your primary focus is Intrinsic Electronic Transport: Choose h-BN to utilize its atomically flat, inert surface and minimize charge impurity scattering.
- If your primary focus is Crystal Quality: Select h-BN to leverage surface edges and wrinkles as nucleation sites for superior epitaxial alignment.
Switching to h-BN transforms the substrate from a passive mechanical support into an active component that enhances both crystal quality and electronic performance.
Summary Table:
| Feature | Silicon Dioxide (SiO2) | Hexagonal Boron Nitride (h-BN) |
|---|---|---|
| Surface Profile | Amorphous & rough | Atomically flat van der Waals surface |
| Chemical State | Contains dangling bonds/traps | Chemically inert |
| Scattering | High charge impurity scattering | Minimal scattering (preserves intrinsic properties) |
| Growth Mechanism | Random nucleation | Controlled epitaxial growth via surface features |
| Device Impact | Performance degradation | High structural and electronic fidelity |
Elevate Your Material Research with KINTEK Precision
Unlock the full potential of your 2D materials and heterostructures with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous demands of epitaxial growth and high-purity material processing. Whether you are working with h-BN substrates or developing advanced WTe2 devices, our lab high-temp furnaces provide the uniform heating and precise control necessary for superior crystal quality.
Ready to optimize your lab’s efficiency? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- Andrejs Terehovs, Gunta Kunakova. Chemical Vapor Deposition for the Fabrication of WTe<sub>2</sub>/h‐BN Heterostructures. DOI: 10.1002/admi.202500091
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- What is the catalytic mechanism of methane gas conversion in Ni-Co CNT synthesis? Master Carbon Transformation
- What function does a water quenching tank serve in Ni-Ti alloy heat treatment? Lock in Superelasticity & Shape Memory
- What role does a pyrolysis furnace play in preparing graphene nanosheets? Master High-Value Plastic Transformation
- How does a laboratory oven contribute to the hydrothermal treatment of graphene aerogels? Master High-Strength Synthesis
- What type of furnaces are commonly used for sintering? Choose the Right Furnace for Your Process
- How does a temperature-controlled experimental platform support the testing of Mn3O4 memristor stability?
- Why is an electric blast drying oven required for CRP microstructure analysis? Ensure Data Accuracy with Proper Drying
- How does the pre-oxidation process affect high-temperature alloys? Enhancing Surface Integrity for Steam Cracking
